PILLAR 3 - PRODUCTION AND VALORIZATION OF GREEN HYDROGEN
Hydrogen is considered today as the most promising energy carrier of the future, especially when produced from renewable sources. For example, vehicles equipped with fuel cells have much greater range than those equipped with batteries. Hydrogen refueling is fast, but the development of this type of vehicle may be hindered if hydrogen stations are not capable of producing sufficient fuel.
The production of green hydrogen from renewable sources is an important national priority. The government plans to invest over 7 billion euros over a 10-year period to support this initiative. Furthermore, 1.9 billion euros will be dedicated to the development of the hydrogen industry as part of the France 2030 investment plan, representing a total budget of 30 billion euros.
Hydrogen plays a crucial role in enabling the storage of intermittently generated electricity, which helps solve the challenge of constant availability of renewable energy. After obtaining hydrogen through water electrolysis, it can be directly used to power or react with carbon dioxide to produce methane or high-value platform molecules, which is known as "power to gas".
The use of electricity generated from renewable sources enables the production of green hydrogen. This hydrogen can be either used directly or stored in high-pressure bottles, and it can also power fuel cells. The principle of fuel cells is to react hydrogen (H) with oxygen (O) to generate electricity, while emitting only water: zero carbon, no fine particles, and no pollution. The electrical energy provided by the fuel cell can then regulate and stabilize consumption during periods of renewable energy underproduction.
Thus, we have a closed loop: hydrogen → electricity → hydrogen.
This topic is complementary to Pillars 1 and 2 since powering future connected devices (Pillar 2) with micro fuel cells is feasible, and water electrolysis can also be carried out using electricity generated from nuclear power during off-peak hours, referred to as yellow hydrogen (Pillar 4). The temperature of the electrolysis directly affects the amount of additional electricity required to split the water molecule. Since the 2000s, there has been significant research effort to improve the efficiency of solid oxide electrolysis cells (such as PEM or SOEC) by increasing their temperature.
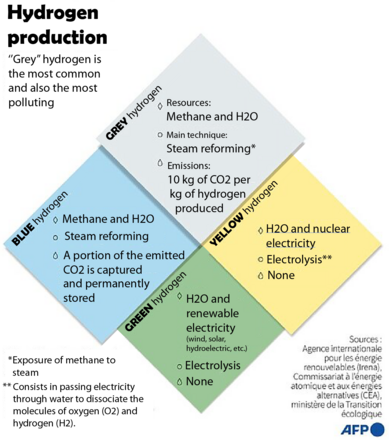
Grey hydrogen comes from methane and H2O, its main technique is steam reforming, and its emissions are 10 kg of CO2 per kg of hydrogen produced. Yellow hydrogen comes from H2O and nuclear electricity, its main technique is electrolysis, and it produces no emissions. Blue hydrogen comes from methane and H2O, its main technique is steam reforming, its emissions are part of the CO2 emitted, but it is captured and permanently stored. Finally, green hydrogen comes from H2O and renewable electricity (wind, solar, hydroelectric, etc.), its main technique is electrolysis, and it produces no emissions.
Objectives and scientific challenges
To this day, there are still a few challenges to overcome, particularly in the development of solid oxide electrolyzers. Besides having higher efficiency compared to the alkaline technologies currently in use, solid oxide electrolyzers have the advantage of enabling the co-electrolysis of water and carbon dioxide, thus creating easily usable synthetic gas. More broadly, managing the intermittency of these devices also presents a hurdle. Lowering operating temperatures and ensuring the durability of these devices are at the forefront of solid-state chemists' research. Co-electrolysis and the production of value-added molecules such as dimethyl ether open up new prospects. Thus, the UCCS (Unité de Catalyse et Chimie du Solide) is currently working on the development of low-temperature, ion-conducting solid oxide fuel cells (ANR BIBELOT) and the rapid research and development of materials for proton-conducting oxide fuel cells (ANR-AUTOMAT-PROCELLS). They are also focusing on developing characterization tools for assessing the degradation modes of these devices as part of a EUROSTAR project led by a Swiss SME, Fiaxell.
The CRIStAL and UCCS laboratories are also involved in an Interreg project that deals with "power to power" and "power to gas," ranging from the management of renewable electricity at the upstream end of the chain (CRIStAL) to the transformation of the produced hydrogen into value-added molecules for the production of innovative fuel (UCCS) (European E2C project, national HYSPAC project).
Furthermore, the management of the electrical grid and mobility aspects are integral to the activities of the L2EP. It is worth mentioning that the CNRS teams are members or affiliates of the Hydrogen Research Federation (FRH2, FR2044), which is led by the CNRS.
Thus, the entire chain, from electricity production to innovative fuel, is covered by the stakeholders at the Lille site.
Task description
The objective is to create a comprehensive hydrogen platform that encompasses the management of electricity from renewable sources (Polytech Lille Multisource Platforms), the development of durable solid oxide fuel cells and electrolyzers (Chevreul Federation Energy Platform), the production of innovative fuels (Realcat Platform), and the study of hybrid vehicle integration (e-V Platform).
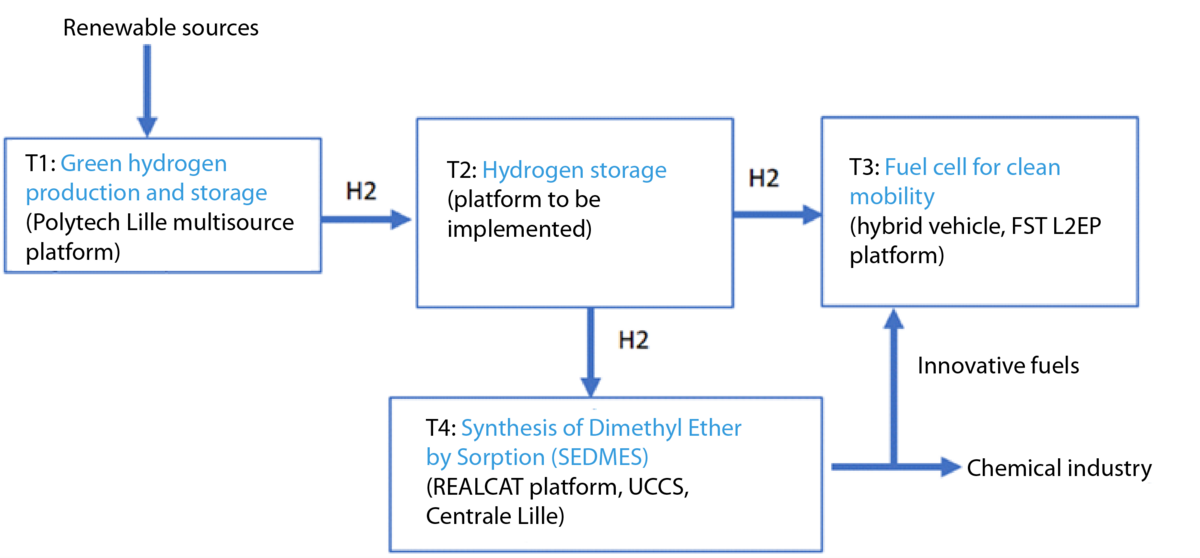
The first task is dedicated to the production and storage of green hydrogen (Polytech Lille Multisources Platform), the second task deals with hydrogen storage (Platform to be set up), the third task focuses on a fuel cell for clean mobility (hybrid vehicle FST L2EP platform), and the fourth and last task is dedicated to the synthesis of Dimethyl Ether by Sorption (Realcat Platform, UCCS, Centrale Lille).
Impacts
Multiple benefits are expected, including:
- Reduction in maintenance costs for multi-source platform equipment (wind turbines, solar panels, electrolyzers, and fuel cells).
- Ability to conduct feasibility tests and techno-economic calculations prior to the implementation of "green" electricity production and storage facilities.
- Decreased costs of decarbonized electricity production.
- Implementation of online monitoring of energy efficiency.
- Production of new chemical products (such as dimethyl ether fuel).
The Hauts-de-France region has an interesting opportunity to develop a hydrogen-focused industry through the establishment of a wind farm and the maintenance of the Gravelines Power Plant. One project, in particular, stands out—the construction of an electrolyzer park. Initially, alkaline technology will likely be used, but considering the increasing demand for hydrogen, solid oxide technology will play a major role in approximately a decade.
The implementation of Master's level programs within the Graduate School of the Lille Engineering Institute opens up new perspectives in key areas such as hydrogen production systems, fuel cell utilization, hydrogen molecule exploitation, control and safety, as well as renewable energies. These specialized training programs aim to educate a new generation of qualified experts, ready to tackle the challenges of the energy transition.